Analysis of S-CA 19-9 Cancer-associated antigen
Venous blood test to analyze the concentration of CA 19-9 which is a tumor marker mainly used to identify and monitor pancreatic and bile duct cancer. By analyzing the CA 19-9 levels, doctors can obtain important information about the occurrence and progression of cancer and follow-up of ongoing treatment. Elevated concentrations above 37 kU/L can also be seen in other forms of cancer and certain benign conditions.
The CA 19-9 test is used in several clinical situations where it is important to assess the presence or treatment of cancer, especially pancreatic cancer. Common situations where the test is recommended include:
- Suspicion of pancreatic cancer
- Follow-up of treatment for pancreatic cancer
- Detected recurrence of pancreatic cancer
Elevated concentrations can also occur at;
- Pancreatic cancer (70-90% of cases)
- Bile duct cancer (up to 80% of cases)
- Colorectal cancer (approx. 30-40% of cases)
- Liver cancer
- Stomach cancer (ventricular cancer)
When is a CA 19-9 test recommended?
Blood test for CA 19-9 is recommended when pancreatic cancer or other cancers of the gastrointestinal tract are suspected. The test is also used to monitor patients who are already being treated for cancer, to assess the effectiveness of the treatment and detect possible recurrences. CA 19-9 levels may be elevated in advanced tumors of the stomach, colon, and bile ducts, as well as in certain non-malignant conditions such as cystic fibrosis, liver cirrhosis, and inflammatory diseases.
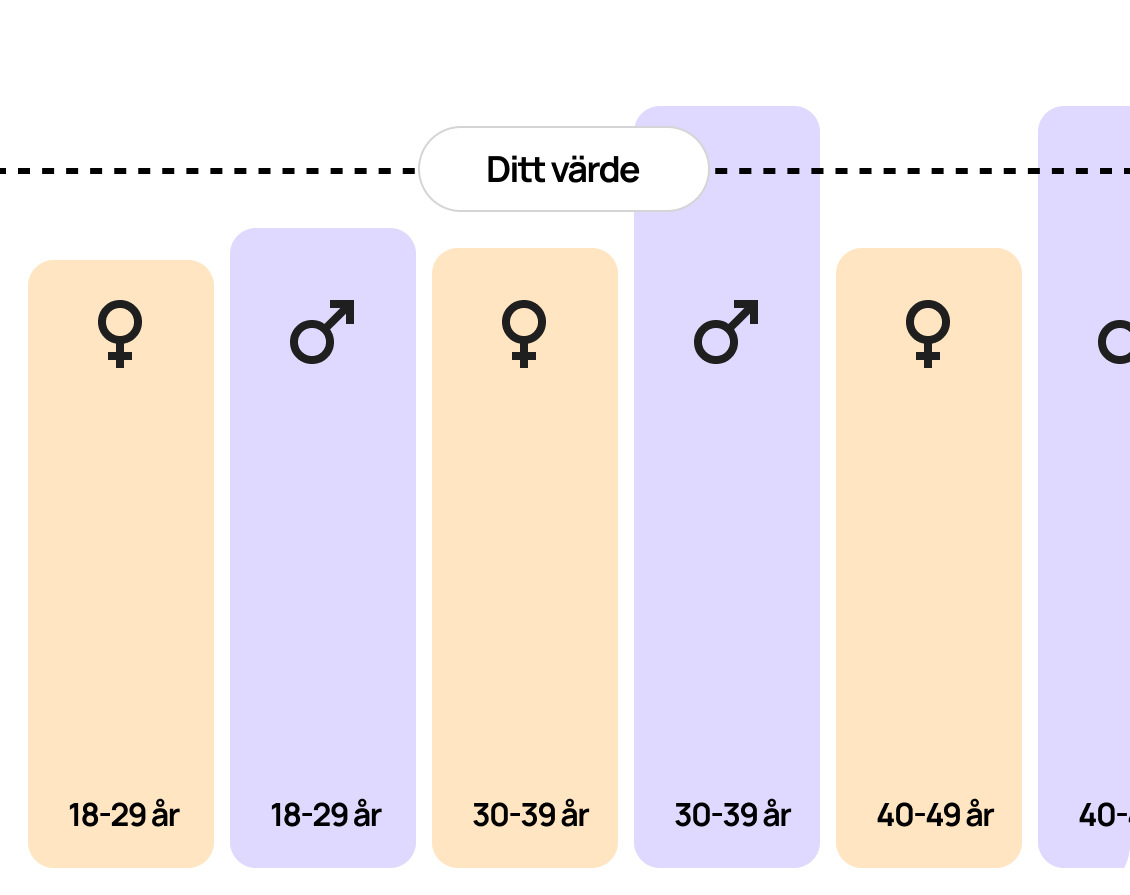
Reference interval
Normal reference values for CA 19-9 are usually below 37 U/mL. Levels above this may indicate the presence of pancreatic cancer or other diseases. It is important to note that reference values may vary depending on the laboratory and the method used to analyze CA 19-9, so the test results should be interpreted with caution.
Interpreting CA 19-9 Test Results
Elevated CA 19-9 levels may indicate cancer of the pancreas, bile ducts, stomach, or colon, but to be clear, it is not possible to make a diagnosis based solely on the results. Mildly elevated levels may occur in noncancerous conditions, such as cystic fibrosis, cirrhosis, primary biliary cirrhosis, cholecystitis, pancreatitis, and rheumatoid arthritis. To get a complete picture, CA 19-9 results should be interpreted in combination with clinical findings and other diagnostic methods, such as imaging, in conjunction with your treating physician.
Important limitations to be aware of
The CA 19-9 test is not appropriate as a screening test for cancer in asymptomatic individuals. It should only be used as part of a broader diagnostic workup or to monitor known cases of cancer. Lack of the Lewis blood group antigen in some individuals may affect the test results, which should be taken into account when interpreting the test results.